Featured
Dupilumab Age Approval
There is a simple discount patient access scheme for dupilumab. PARIS and TARRYTOWN NY.
 Example Of Case Before And After Treatment With Dupilumab 300mg Every Download Scientific Diagram
Example Of Case Before And After Treatment With Dupilumab 300mg Every Download Scientific Diagram
FDA Approves Dupixent dupilumab as First Biologic Medicine for Children Aged 6 to 11 Years with Moderate-to-Severe Atopic Dermatitis.

Dupilumab age approval. Food and Drug Administration FDA approved Dupixent dupilumab for children aged 6 to 11 years with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. And PARIS May 26 2020 PRNewswire --. Approval FDA Approves Dupixent dupilumab for Chronic Rhinosinusitis with.
May 26 2020 - The US. Is this guidance up to date. The recommended dose of DUPIXENT for patients 12 to 17 years of age is specified in Table 1.
And PARIS May 26 2020 PRNewswire --. In addition to the currently approved indications Regeneron and Sanofi are also studying dupilumab in a broad range of clinical development programs for diseases driven by allergic and other type 2 inflammation including pediatric asthma 6 to 11 years of age Phase 3 pediatric atopic dermatitis 6 months to 5 years of age Phase 23 eosinophilic esophagitis Phase 3. Food and Drug Administration FDA approved Dupixent dupilumab for children aged 6 to 11 years with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
And TARRYTOWN NY March 11 2019 PRNewswire -- The US. FDA Approves Dupixent dupilumab as First Biologic Medicine for Children Aged 6 to 11 Years with Moderate-to-Severe Atopic Dermatitis. Dupixent is the first and only biologic approved for the treatment of uncontrolled moderate-to-severe atopic dermatitis for people 12 years of age and older in the EU and 6 years of age and older in the US.
CHMP Recommends Approval of Dupixent dupilumab for Children Aged 6 to 11 Years with Severe Atopic Dermatitis. In children aged 6-11 years weighing 15 to. Only biologic approved for the treatment of uncontrolled moderate-to-severe atopic dermatitis for people 12 years of age and older in.
In the pivotal trial more than twice as many children achieved clear or almost clear skin and more than four times achieved itch reduction with Dupixent. Dupilumab in the treatment of children aged 6 to 11 years of age with severe atopic dermatitis allergic eczema EAMS scientific opinion issued to Sanofi for dupilumab in the treatment of children. Dose of DUPIXENT for Subcutaneous Administration in Adolescent Patients Body.
Approval FDA Approves Dupixent dupilumab as First Biologic Medicine for Children Aged 6 to 11 Years with Moderate-to-Severe Atopic Dermatitis. PARIS and TARRYTOWN NY August 6 2019 - The European Commission EC today extended the marketing authorization for Dupixent dupilumab in the European Union EU to include adolescents 12 to 17 years of age with moderate-to-severe atopic dermatitis who are candidates for systemic therapy. Evidence-based recommendations on dupilumab Dupixent for treating moderate to severe atopic dermatitis in adults.
Dupixent is also approved in the EU for certain patients with severe asthma and severe chronic rhinosinusitis with nasal polyps CRSwNP two other type 2. Dupilumab Dupixent a monoclonal antibody directed against interleukins-4 and -13 initially approved in adults received an expanded indication from the Food and Drug Administration in March 2019 for treatment of 12- to 17-year-olds with moderate to severe atopic dermatitis AD on the strength of a pivotal 251-patient phase 3 randomized trial of 16 weeks. FDA has approved dupilumab Dupixent Sanofi and Regeneron for the treatment of atopic dermatitis in children 6-11 years making it the first biologic approved for atopic dermatitis in this particular age group.
Recommendation based on pivotal trial that showed Dupixent plus topical corticosteroids TCS significantly improved measures of overall disease severity skin clearance itch and health-related quality of life compared to TCS alone. Food and Drug Administration FDA has approved Dupixent dupilumab for adolescent patients 12 to 17 years of age with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not. Dupixent is the first and only biologic approved for the treatment of uncontrolled moderate-to-severe atopic dermatitis for people 12 years of age and older in the EU and 6 years of age and older.
CHMP Recommends Approval of Dupixent dupilumab for.
 On A Roll Dupixent Marches Into Teen Eczema Nabs Fast Fda Review In Sinusitis Fiercepharma
On A Roll Dupixent Marches Into Teen Eczema Nabs Fast Fda Review In Sinusitis Fiercepharma
 Fda Approves Dupixent Dupilumab As First Biologic Medicine For Children Aged 6 To 11 Years
Fda Approves Dupixent Dupilumab As First Biologic Medicine For Children Aged 6 To 11 Years
 Dupixent Dupilumab For Moderate To Severe Eczema That Is Uncontrolled
Dupixent Dupilumab For Moderate To Severe Eczema That Is Uncontrolled
 Fda Approves Dupixent For Children With Moderate To Severe Atopic Dermatitis Pharmalive
Fda Approves Dupixent For Children With Moderate To Severe Atopic Dermatitis Pharmalive
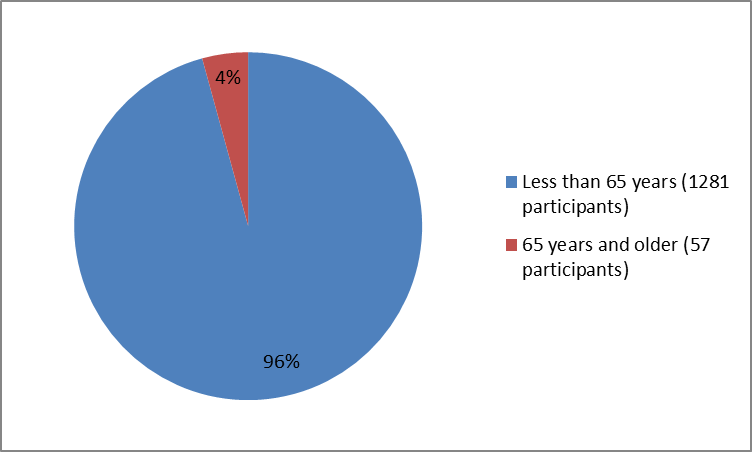 Drug Trials Snapshots Dupixent Fda
Drug Trials Snapshots Dupixent Fda
![]() Regeneron Pharmaceuticals Fda Approves Dupixent Dupilumab As First Biologic Medicine For Children Aged 6 To 11 Years With Moderate To Severe Atopic Dermatitis Fda Health News
Regeneron Pharmaceuticals Fda Approves Dupixent Dupilumab As First Biologic Medicine For Children Aged 6 To 11 Years With Moderate To Severe Atopic Dermatitis Fda Health News
 Fda Approves Dupixent Dupilumab For Moderate To Severe Atopic Dermatitis In Adolescents Snacksafely Com
Fda Approves Dupixent Dupilumab For Moderate To Severe Atopic Dermatitis In Adolescents Snacksafely Com
 Fda Approves Dupixent For Treatment Of Asthma Asthma And Allergy Foundation Of America
Fda Approves Dupixent For Treatment Of Asthma Asthma And Allergy Foundation Of America
 Dupixent Approval Expanded To Pediatric Patients With Atopic Dermatitis
Dupixent Approval Expanded To Pediatric Patients With Atopic Dermatitis
 Regeneron Pharmaceuticals Inc Fda Accepts For Priority Review Dupixent Dupilumab For Children Aged 6 To 11 Years With Moderate To Severe Atopic Dermatitis Fda Health News
Regeneron Pharmaceuticals Inc Fda Accepts For Priority Review Dupixent Dupilumab For Children Aged 6 To 11 Years With Moderate To Severe Atopic Dermatitis Fda Health News
 Pdf Efficacy And Safety Of Dupilumab With Concomitant Topical Corticosteroids In Children 6 To 11 Years Old With Severe Atopic Dermatitis A Randomized Double Blinded Placebo Controlled Phase 3 Trial
Pdf Efficacy And Safety Of Dupilumab With Concomitant Topical Corticosteroids In Children 6 To 11 Years Old With Severe Atopic Dermatitis A Randomized Double Blinded Placebo Controlled Phase 3 Trial
 Lanny Rosenwasser On Twitter Dupilumab Approved For Age 6 11 In Atopic Dermatitis Data Are In And Pending For 6 11 Asthma Approval May Follow Should Outperform Omalizumab Https T Co Fgbwaxo6nx
Lanny Rosenwasser On Twitter Dupilumab Approved For Age 6 11 In Atopic Dermatitis Data Are In And Pending For 6 11 Asthma Approval May Follow Should Outperform Omalizumab Https T Co Fgbwaxo6nx

Comments
Post a Comment