Featured
Emd Serono Ms Drug
Still the group fell short on delivering its German bosses an FDA approval of cladribine an oral drug for relapsing-remitting cases of MS. 7588755 wasnt anticipated by prior art.
 Fda Approves Mavenclad Cladribine Tablets For Multiple Sclerosis
Fda Approves Mavenclad Cladribine Tablets For Multiple Sclerosis
Merck KGaA Darmstadt Germany which operates this website uses the firm name Merck KGaA Darmstadt Germany in the United States and Canada and also uses EMD Serono in biopharma MilliporeSigma in life science and EMD Performance Materials in materials business.
Emd serono ms drug. Makes no claims that the Materials may be lawfully viewed or downloaded outside of the United States. Leaders in Multiple Sclerosis. EMD Serono a unit of Merck KGaA Darmstadt Germany announced that the US.
It is indicated as monotherapy for the treatment of adult patients with relapsing-remitting multiple sclerosis RRMS to reduce the frequency of clinical exacerbations and delay the progression of disability. Mavenclad is the first and only treatment for RRMS and active SPMS approved by the. Always consult a physician if you have health concerns.
Emd serono MS Lifelines Mavenclad cladribine tablet. The three-judge panel reversed US. The drug is not recommended for MS patients with a course of the disease known as clinically isolated syndrome.
The precedential ruling is a victory for. Food and Drug Administration FDA had approved Mavenclad cladribine for adults with relapsing-remitting multiple sclerosis RRMS and active secondary progressive disease SPMS. This site contains information that is intended for residents of the United States only and is not meant to substitute for the advice provided by a medical professional.
District Judge Claire C. The agency handed EMD Serono a complete response on its. EMD Serono MS drug delayed The US Food and Drug Administration extended the review period of an EMD Serono multiple sclerosis drug by three months The US Food and Drug Administration FDA has pushed back the date by which it will make its decision on a marketing application for a new multiple sclerosis MS drug filed by EMD Serono an.
MAVENCLAD also known as cladribine is an oral selective Immunosuppressant. Due to safety concerns Mavenclad is generally recommended for patients who have not. EMD Serono a Division of EMD Serono Canada Inc.
Use and access of this site is subject to the terms and conditions as set. EMD Seronos experience and scientific discoveries in MS date back more than 20 years. Food and Drug Administration today approved Mavenclad cladribine tablets to treat relapsing forms of multiple sclerosis MS in adults to include relapsing-remitting disease and active secondary progressive disease.
Cladribine MavencladEMD Serono Cladribine is a purine antimetabolite oral tablet that reduces the damaging immune responsein which the immune system mistakenly attacks the CNSseen in MS. Fernando Dangond MD MBA EMD Seronos investigational oral highly selective Bruton tyrosine kinase BTK inhibitor evobrutinib is the focus of 2 pivotal phase 3 trials in relapsing multiple sclerosis MS that are expected to conclude in September 2023. Mavenclad is not recommended for MS patients with clinically isolated syndrome.
EMD Serono the North American biopharmaceutical business arm of Merck announced it has gained exclusive rights over Rebif interferon beta-1a in the United States. Because of its safety profile the use of Mavenclad is generally recommended for patients who have had an inadequate response to or are unable to tolerate an alternate drug. Prime Therapeutics LLC Prime a leading pharmacy benefit manager PBM serving more than 30 million members nationally signed a value-based agreement with EMD Serono for its oral multiple sclerosis MS therapy MAVENCLAD cladribine tablets which was approved by the Food and Drug Administration FDA in 2019.
FDA issues CRL to EMD Seronos MS drug articleStaff2011FDAIC titleFDA issues CRL to EMD Seronos MS drug authorFormulary Staff journalFormulary year2011 Formulary Staff. FDA issued a complete response letter to EMD Serono an affiliate of Merck KGaA requesting additional information on cladribine Movectro a drug. Rebif is a treatment for.
Because there are no data on the effect of interferon beta-1a on milk production EMD Serono emphasized that the developmental and health benefits of breastfeeding should be considered as well as. To this end we offer delivery devices for our therapeutic products as well as comprehensive patient education resources. EMD Serono is the biopharmaceutical business of Merck KGaA Darmstadt Germany.
Will provide notice to you promptly of any such claim suit or proceeding and will assist you at your expense in defending any such claim suit or proceeding. Access to the Materials may not be legal by certain persons or in certain. For EMD Serono maker of longtime MS standby Rebif and newer therapy Mavenclad the added value of a social network is the opportunity to listen to MS patients and to identify their concerns and.
Cecchis 2018 judgment holding that US. There are two different unaffiliated companies that use the name MERCK. We have an ongoing commitment to improving the entire patient experience.
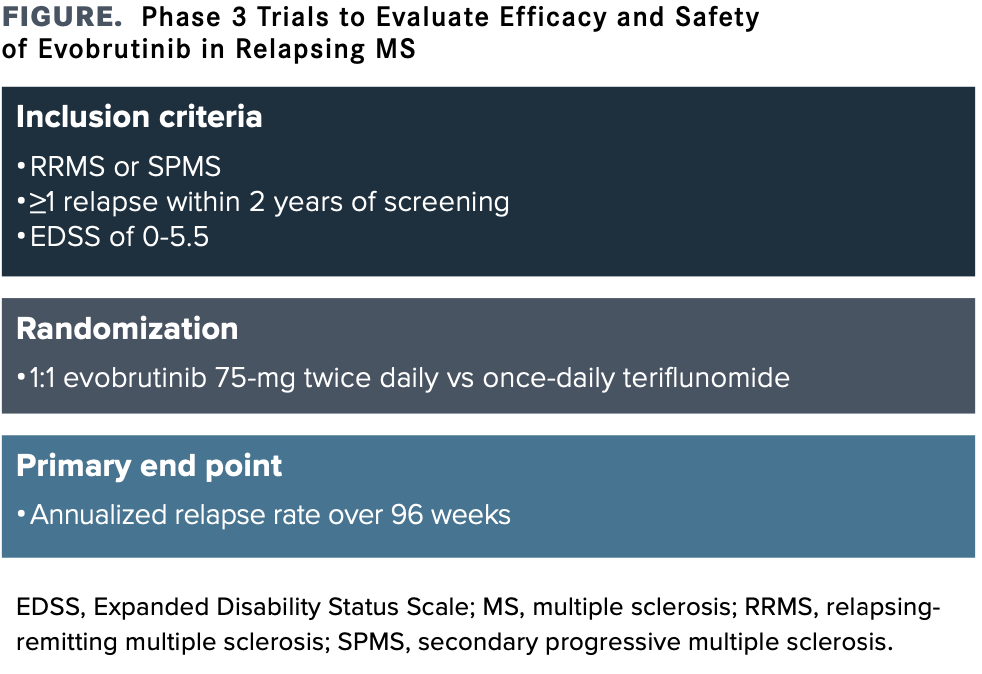 Evobrutinib Paves The Way For Btk Inhibitors In Multiple Sclerosis
Evobrutinib Paves The Way For Btk Inhibitors In Multiple Sclerosis
Fda Green Lights Emd Serono S Mavenclad For Multiple Sclerosis In The U S Biospace
 Fda Approves Mavenclad Cladribine Tablets For Multiple Sclerosis
Fda Approves Mavenclad Cladribine Tablets For Multiple Sclerosis
 Emd Serono And The Pan Canadian Pharmaceutical Alliance Complete Negotiations For Mavenclad Cladribine Tablets For The Treatment Of Relapsing Remitting Multiple Sclerosis
Emd Serono And The Pan Canadian Pharmaceutical Alliance Complete Negotiations For Mavenclad Cladribine Tablets For The Treatment Of Relapsing Remitting Multiple Sclerosis
Fda Knocks Back Merck Kgaa S Oral Ms Drug Pharmafile
 Emd Serono Announces New Data Strengthening Evidence For Continued Safe And Effective Mavenclad Use During The Covid 19 Pandemic Business Wire
Emd Serono Announces New Data Strengthening Evidence For Continued Safe And Effective Mavenclad Use During The Covid 19 Pandemic Business Wire
 Cadth Canadian Drug Expert Committee Recommendation Cladribine Mavenclad Emd Serono Ncbi Bookshelf
Cadth Canadian Drug Expert Committee Recommendation Cladribine Mavenclad Emd Serono Ncbi Bookshelf
Https Www Merckgroup Com Content Dam Web Corporate Non Images Investors Events And Presentations Webcasts And Presentations 2020 En 2020 Evobrutinib Strategy Update En Pdf
 Accelerated Cure Project Collaborates With Emd Serono To Advance Patient Focused Drug Development In Multiple Sclerosis Specialty Pharma Journal
Accelerated Cure Project Collaborates With Emd Serono To Advance Patient Focused Drug Development In Multiple Sclerosis Specialty Pharma Journal
 Fda Approves Cladribine Mavenclad For Relapsing Ms
Fda Approves Cladribine Mavenclad For Relapsing Ms
 Merck Kgaa Myhealthteams Create Social Media Resource Center Ms Patients Fiercepharma
Merck Kgaa Myhealthteams Create Social Media Resource Center Ms Patients Fiercepharma
 Emd Serono Gains Exclusive Us Rights To Ms Drug For Relapsing Forms Of Disease Multiple Sclerosis News Today
Emd Serono Gains Exclusive Us Rights To Ms Drug For Relapsing Forms Of Disease Multiple Sclerosis News Today


Comments
Post a Comment