Featured
Voxelotor Fda Label
Voxelotor was granted accelerated approval by the US. Voxelotor a first-in-class drug is currently being studied for the treat- ment of sickle cell disease in the United States and Europe that has received Breakthrough Therapy Designation BTD by the Food and Drug Administration FDA and the European Medicines Agency Prior-ity Medicines PRIME designation.
Advise not to breastfeed 82.

Voxelotor fda label. Approval Date s and History Letters Labels Reviews for NDA 213137. A Hemoglobin S Polymerization Inhibitor for the Treatment of Sickle Cell Disease November 2020 Journal of the Advanced Practitioner in Oncology 118. 11 CD33-positive Acute Myeloid Leukemia AML 12 Relapsed or Refractory.
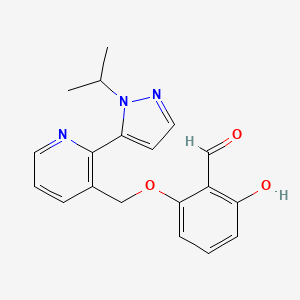
Voxelotor is a hemoglobin S polymerization inhibitor. HEPATOTOXICITY 1 INDICATIONS AND USAGE. Voxelotor has a molecular formula of C 19 H 19 N 3 O 3 and a molecular.
FDA approves voxelotor for sickle cell disease FDA FDA approves voxelotor for sickle cell disease On November 25 2019 the Food and Drug Administration granted accelerated approval to voxelotor. Emmaus Medicals Endari was approved in 2017 as the first new sickle cell treatment in 20 years. Approval comes just 10 days after the FDA cleared Novartis Adakveo for use in the same condition.
The average plasma half-life of voxelotor was 50 hours in patients with sickle cell disease compared with 6185 hours in healthy patients in one clinical studyA188126. Original Approvals or Tentative Approvals. As of December 2017 there are three published manuscripts on.
For Oxbryta voxelotor tablets for oral use. Open-label extension OLE study of voxelotor for pediatric participants with Sickle Cell Disease who have participated in voxelotor clinical trials. Auch für Europa ist die Zulassung beantragt.
Die US-amerikanische Gesundheitsbehörde FDA hat einen neuen Wirkstoff bei Sichelzell-Anämie beschleunigt zugelassen. The plasma elimination half-life of voxelotor in sickle cell disease patients is about 355 hours according to the FDA labelL10397 The mean half-life in the red blood cell is 60 days. Voxelotor verhindert die Polymerisation des verformten Hämoglobins S und erhöht dadurch im Vergleich zu Placebo signifikant den Hämoglobin-Wert der Betroffenen.
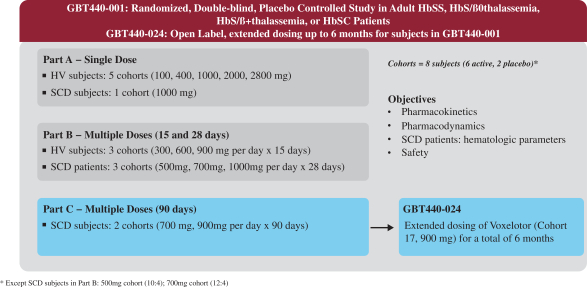
All participants will receive voxelotor once daily administered orally as tablets. The FDA granted the application for voxelotor fast. 454 Zeilen The table below lists therapeutic products from DrugsFDA with.
1-800-438-1985 or FDA at 1-800-FDA-1088 or wwwfdagovmedwatch. See 17 for PATIENT COUNSELING INFORMATION. This study will assess exercise capacity by cardiopulmonary exercise.
The chemical name of voxelotor is. With global blood therapeutics having already received US FDA approval in November 2019 voxelotor may soon be an addition to the mounting armoury of drugs against SCD. Approximately 50 participants with sickle cell disease SCD aged 4 to 18 years will be enrolled at approximately 19 global clinical sites.
Letters Reviews Labels Patient Package Insert. This study is a pilot open-label single-arm study to evaluate the effect of the sickle cell medication voxelotor on exercise capacity as measured by cardiopulmonary exercise testing CPET in patients 12 years of age and older with sickle cell anemia SCA. Assessment for vox elotor for patients with sickle cell disease.
Food and Drug Administration FDA in November 2019 and further clinical trials are required to verify and describe Oxbrytas clinical benefit. Voxelotor which will be sold as Oxbryta will be available within two weeks the company said. The labeling adequately addresses known risks and the Applicant intends to confirm and verify clinical benefit with an.
In response to DHPs consult request dated July.
Fda Approves Oxbryta Voxelotor The First Medicine
 Press Release Gbt Announces The Fda Agrees To Submit A New Drug Application For The Approval Of Voxelotor Sickle Cell Disease Association Of America Inc
Press Release Gbt Announces The Fda Agrees To Submit A New Drug Application For The Approval Of Voxelotor Sickle Cell Disease Association Of America Inc
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2019 213137s000lbl Pdf
 Fda Approves Oxbryta Voxelotor The First Medicine Specifically Targeting The Root Cause Of Sickle Cell Disease Tif
Fda Approves Oxbryta Voxelotor The First Medicine Specifically Targeting The Root Cause Of Sickle Cell Disease Tif
 Providing Hope To The Underserved Ppt Download
Providing Hope To The Underserved Ppt Download
 Ndc 72786 101 Oxbryta Voxelotor
Ndc 72786 101 Oxbryta Voxelotor
 Press Release Gbt Announces The Fda Agrees To Submit A New Drug Application For The Approval Of Voxelotor Sickle Cell Disease Association Of America Inc
Press Release Gbt Announces The Fda Agrees To Submit A New Drug Application For The Approval Of Voxelotor Sickle Cell Disease Association Of America Inc
 Pdf Voxelotor A Ray Of Hope For Sickle Disease
Pdf Voxelotor A Ray Of Hope For Sickle Disease
 Accelerated Approval Of Oxbryta Voxelotor A Case Study On Novel Endpoint Selection In Sickle Cell Disease Sciencedirect
Accelerated Approval Of Oxbryta Voxelotor A Case Study On Novel Endpoint Selection In Sickle Cell Disease Sciencedirect
 Fda Approves Oxbryta Voxelotor For The Treatment Of Sickle Cell Disease Cliniexpert
Fda Approves Oxbryta Voxelotor For The Treatment Of Sickle Cell Disease Cliniexpert
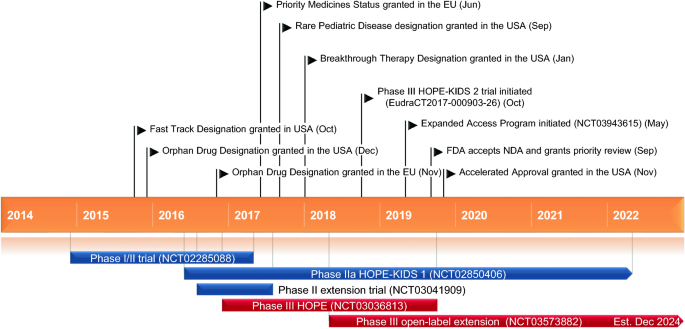 Voxelotor First Approval Springerlink
Voxelotor First Approval Springerlink
Comments
Post a Comment